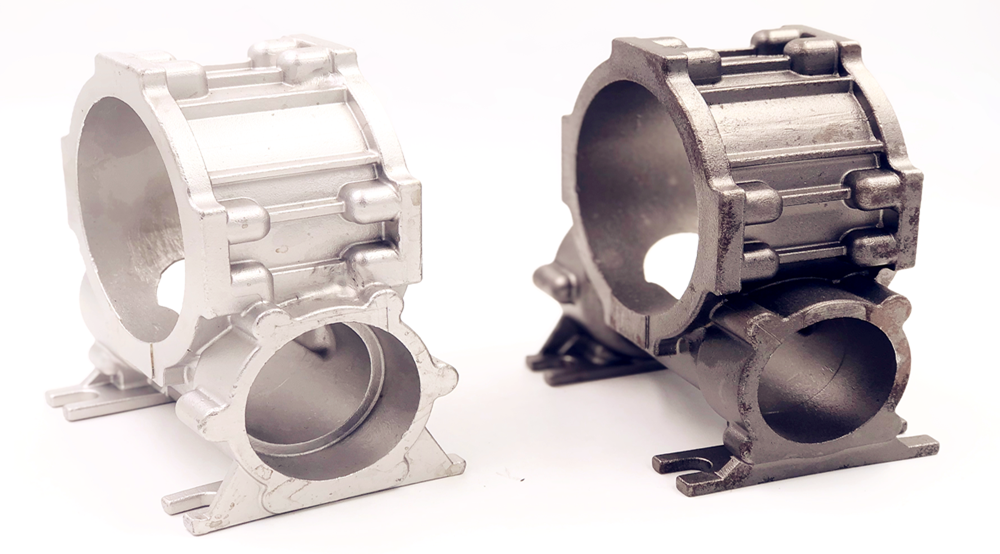
The method of removing scale and rust from the surface of steel using an acid solution is called pickling. It is a technique for cleaning metal surfaces and is usually performed in conjunction with pre-passivation treatment.
Typically, parts are immersed in an aqueous solution (such as sulfuric acid) to remove the oxide film on the metal surface. It serves as a pre-treatment or intermediate treatment for processes like electroplating, enameling, and rolling.
If the corrosion products generated on a metal due to the action of a medium have a dense structure and form a film (usually invisible) that tightly covers the metal surface, the surface state of the metal changes: the electrode potential of the metal increases significantly (jumps in the positive direction), transforming the metal into a corrosion-resistant passive state.
For example, the standard potential of iron (when iron is oxidized to Fe²⁺) is -0.44V. After passivation, this potential jumps to 0.5–1V, and the iron exhibits corrosion resistance similar to that of noble metals. This film is called a passivation film.
Metal passivation can also occur as a spontaneous process (e.g., the formation of an insoluble compound film, i.e., an oxide film, on the metal surface). Industrially, passivators (primarily oxidizing agents) are used to passivate metals and form a protective film.Common examples: Iron and aluminum can both be passivated by cold concentrated sulfuric acid or cold concentrated nitric acid.
The main and only purpose of pickling is to remove the scale on the surface of stainless steel castings. Therefore, pickling is not mandatory for all stainless steel castings—it is only necessary when scale is present on the surface. For instance, stainless steel castings annealed with protective gases (such as pure hydrogen and ammonia decomposition products) do not require pickling.
Pickling of stainless steel is usually associated with passivation. Stainless steel castings that require pickling generally need to be passivated, because no passivation film is formed on the surface after pickling, or the formed passivation film is too thin to be effective. A subsequent passivation process must be added to form and perfect the passivation film on the stainless steel surface. This film is the key to preventing stainless steel from rusting and corroding in general media.
Compared with traditional physical sealing methods, passivation treatment has the characteristics of not increasing the thickness of the workpiece at all and no discoloration. It improves the precision and added value of the product and makes operation more convenient.
Since the passivation process is carried out in a non-reactive state, the passivator can be added repeatedly and reused, resulting in a longer service life and more economical cost.
Passivation promotes the formation of an oxygen-molecule-structured passivation film on the metal surface. This film is dense and stable in performance, and has a self-repairing effect in air. Therefore, compared with the traditional anti-rust method of applying anti-rust oil, the passivation film formed by the passivation layer is more stable and corrosion-resistant.
Hang the stainless steel parts → Chemical degreasing (conventional alkaline chemical degreasing or surfactant degreasing) → Hot water rinsing → Tap water rinsing → First pickling → Tap water rinsing → Second pickling → Tap water rinsing → Transfer to the next process (e.g.: Chemical coloring → Recycling → Tap water rinsing → Hardening treatment → Tap water rinsing → Sealing treatment → Tap water rinsing → Drying → Finished product).
Before pickling and passivation of stainless steel workpieces, if there is surface dirt, mechanical cleaning should be carried out first, followed by degreasing. If the pickling solution and passivation solution cannot remove oil, the presence of oil on the surface will affect the quality of pickling and passivation. Therefore, degreasing cannot be omitted, and alkaline solutions, emulsifiers, organic solvents, or steam can be used for degreasing.